
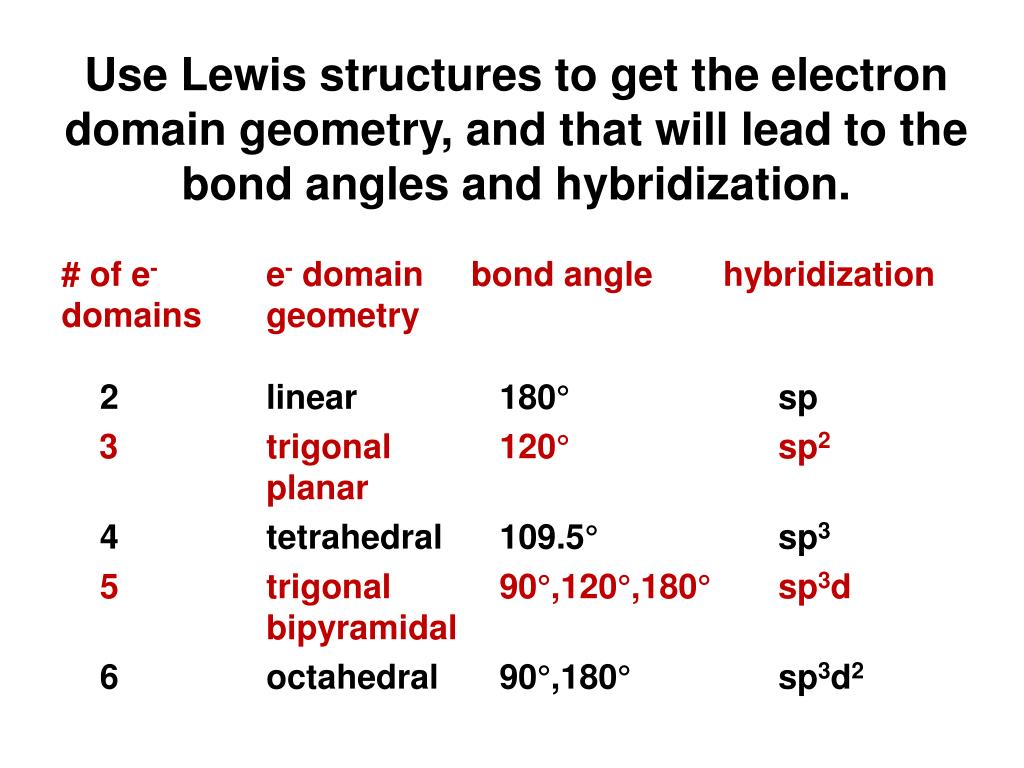
Orbitals are a model representation of the behavior of electrons within molecules. The amount of p character or s character, which is decided mainly by orbital hybridisation, can be used to reliably predict molecular properties such as acidity or basicity. Hybridisation theory explains bonding in alkenes and methane. For drawing reaction mechanisms sometimes a classical bonding picture is needed with two atoms sharing two electrons.

Hybridisation theory is an integral part of organic chemistry, one of the most compelling examples being Baldwin's rules. It gives a simple orbital picture equivalent to Lewis structures. This concept was developed for such simple chemical systems, but the approach was later applied more widely, and today it is considered an effective heuristic for rationalizing the structures of organic compounds. Each hybrid is denoted sp 3 to indicate its composition, and is directed along one of the four C-H bonds. Pauling supposed that in the presence of four hydrogen atoms, the s and p orbitals form four equivalent combinations which he called hybrid orbitals. The angle between any two bonds is the tetrahedral bond angle of 109☂8' (approx. In reality, methane has four C-H bonds of equivalent strength. Pauling pointed out that a carbon atom forms four bonds by using one s and three p orbitals, so that "it might be inferred" that a carbon atom would form three bonds at right angles (using p orbitals) and a fourth weaker bond using the s orbital in some arbitrary direction.


 0 kommentar(er)
0 kommentar(er)
